LOS ANGELES (Reuters): Blood tests for Alzheimer’s are needed to more widely diagnose the brain-wasting disease and understand its prevalence, but it will be another couple of years before they become an everyday tool, medical experts and company executives say.
Blood testing is initially likely to be used to rule out Alzheimer’s, with positive results signalling the need for more advanced diagnostics.
Several Alzheimer’s blood tests are in the works – and one is already being sold to consumers – but none have been established as accurate, formally approved by regulators or reimbursed by insurers. Some are being used to help screen participants enrolling in clinical trials of Alzheimer’s treatments.
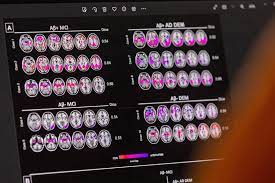
Alzheimer’s, which gradually destroys memory and thinking skills, is characterized by changes in the brain including build-up of amyloid beta plaques and tau tangles that result in loss of neurons responsible for transmitting information.
Currently, people who might benefit from Leqembi, the new Alzheimer’s drug from Eisai and Biogen, need those changes diagnosed through cognitive assessment and a cerebrospinal fluid (CSF) test, which requires an invasive lumbar puncture, or an expensive PET brain scan that may not be reimbursed by health insurers.
Even when covered, CSF and PET testing can be hard to access in some parts of the country.
Researchers have been working for years on blood tests for Alzheimer’s that can replicate these diagnostic tools. But there is still debate over which biomarkers, or proteins in the blood, signal the presence of amyloid and tau in the brain.
They also say more work is needed to understand the relationship of such biomarkers with race, underlying medical conditions and other factors.
The need for blood tests has become more pressing since the FDA approved Leqembi in July. It is currently reviewing a similar drug, donanemab, from Eli Lilly.
Dr Sarah Kremen, director of the neurobehaviour programme at Cedars-Sinai Medical Center in Los Angeles, said amyloid on its own is an unreliable marker for Alzheimer’s since many people accumulate brain deposits of the protein as they age, but do not develop dementia.
Blood tests that measure the right combination of biomarkers will eventually become the standard for diagnosing Alzheimer’s, she said, “but right now, they are not quite ready for prime time.”
NEW COMBINATION TEST ARRIVES
Labcorp on Wednesday began offering to physicians a blood test it says measures markers for amyloid, tau and neurodegeneration with a list price of $626.
In July, Quest Diagnostics launched the first direct-to-consumer blood test for Alzheimer’s, which costs $399 and aims to detect abnormal levels of amyloid. Data on its accuracy has not been published.
Cedars and other neurology centers took the unusual step of issuing strongly-worded cautions about such consumer tests for people who do not have risk factors or symptoms of Alzheimer’s, saying they are best used in conjunction with medical guidance.
“The blood biomarker is a useful screening tool at first, but additional information is needed,” said Eliezer Masliah, director of the National Institute on Aging neuroscience division, part of the US National Institutes of Health.
Eisai has a collaboration with C2N Diagnostics to develop evidence supporting the use of blood-based Alzheimer’s testing.
Michael Irizarry, head of clinical research at Eisai’s neurology division, said he expects the transition to blood-based Alzheimer’s diagnostics to happen over the next two years. “Blood tests are progressing quite rapidly,” he said.
C2N, which has published and presented its data at medical conferences, recently began offering a $1,450 updated version of its blood test that measures amyloid and tau.
Eli Lilly is working with Quanterix on an experimental tau biomarker blood test, and has partnered with Roche to develop a test measuring blood levels of a different tau protein as well as a gene associated with Alzheimer’s risk.
Bruce Jordan, an executive with Roche Diagnostics, said the company expects to have results from a large trial and file for US approval of its Elecsys Amyloid Plasma Panel in 2025.
Accurate blood tests are expected to help identify which dementia patients actually have Alzheimer’s, the most common but not the only cause of dementia.
The Alzheimer’s Association estimates that 6.7 million Americans have the disease, but acknowledges that the number could be 30% lower if biological testing is used.
Another analysis from RAND found that the US. age-adjusted prevalence of dementia fell by about a third to 8.5 per cent between 2000 and 2016, possibly due to more education, less smoking and better heart health care.
“There is a real possibility that the field changes as real world evidence is collected,” said Russ Paulsen, chief operating officer at patient advocacy group UsAgainstAlzheimers.
“When there are widely available, scalable, sensitive and specific blood tests it will be an absolute game changer for Alzheimer’s patients.”