LONDON (Reuters) : The European Union’s medicines regulator has delayed it decision on Eisai and partner Biogen’s Alzheimer’s disease drug that was expected this week, the Japanese company said on Friday.
On Monday, the European Medicines Agency had said it would hold a oral hearing to discuss the drug, lecanemab, this week.
Eisai said the delay was due to a EU court ruling last week, unrelated to the Alzheimer’s drug, that had “implications on EMA’s policy on the handling of competing interests of experts,” Eisai said.
The EMA will schedule a new meeting of its experts in the future to decide on the drug, Eisai said.
“The evaluation of lecanemab is still ongoing,” the EMA said, adding that it would communicate the decision when it had one.
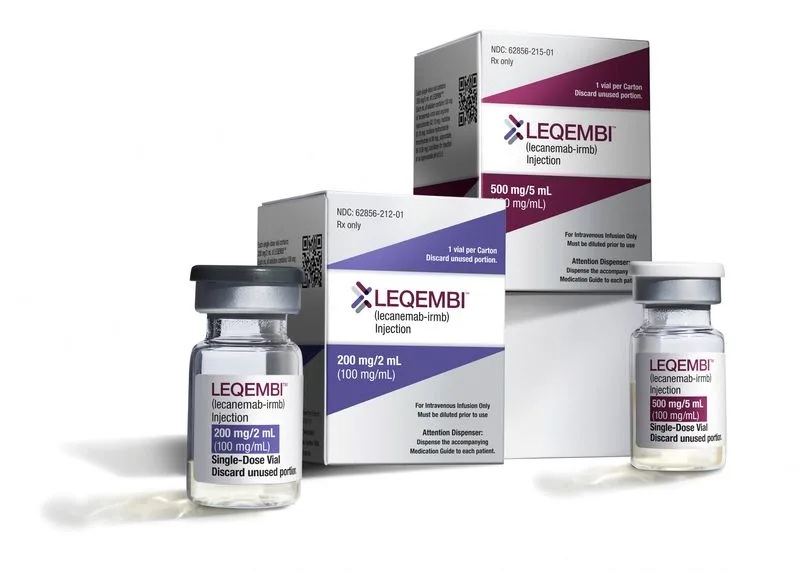
The drug, sold as Leqembi in the United States has been under review in Europe since January 2023. It gained traditional approval in the United States last year and is also approved in other countries, including China and Japan.
In Europe, 7 million people are living with the brain-wasting disease, and that figure is expected to double by 2050, according to Alzheimer’s Europe.
The infusion given twice a month removes sticky clumps of a protein called amyloid beta, believed to be a hallmark of Alzheimer’s, from the brain.